QU’EST-CE QUE L’HEXANE?
Découvrir ses effets cachés
Des études récentes menées en France et en Italie ont mis en évidence la présence inquiétante de traces de n-hexane dans l’organisme de personnes venant de plusieurs pays.
Cette page explique d’où vient cette substance et quels risques pour la santé elle peut représenter.
Des chercheurs alertent sur les risques liés à l’hexane
Des équipes universitaires en France et en Italie ont montré que la population générale est largement exposée à l’hexane. Ce solvant est connu pour être toxique pour le système nerveux.
L’analyse de plusieurs études menées au Mexique, en Italie, en Chine, en Suède, au Japon et en Allemagne confirme la présence de métabolites de n-hexane chez de nombreuses personnes.
D’où vient l’hexane présent dans notre quotidien?
On estime que cette exposition provient en grande partie de résidus dans les aliments, qu’ils soient d’origine végétale ou animale.
L’hexane est utilisé comme auxiliaire de fabrication dans certains procédés alimentaires, pour l’alimentation animale ou encore dans l’industrie pharmaceutique. Une fois utilisé, il est difficile d’en éliminer totalement les traces.
Quels sont les effets possibles sur la santé ?
Les impacts de l’hexane ont été étudiés dans différents contextes : enquêtes de santé au travail, observations en population générale et recherches en laboratoire.
Ces travaux indiquent que les effets neurologiques, respiratoires, sur le développement et la reproduction figurent parmi les principales préoccupations de santé liées à l’exposition à l’hexane.
Qu’est-ce que l’hexane ?
L’hexane ou n-hexane est un alcane à chaîne linéaire composé de six atomes de carbone, de formule moléculaire C6H14. C’est un liquide incolore, avec une odeur légère mais désagréable, rappelant l’essence. Le n-hexane est produit à partir du gaz naturel et du pétrole brut. Son principal usage industriel est celui de solvant.
L’hexane commercial est un mélange contenant environ 52 % de n-hexane. Le reste est constitué de proportions variables d’isomères structurels et de composés apparentés, comme le méthylpentane ou le méthylcyclopentane.
À quel point l’hexane est-il dangereux ?
Identification des dangers par l’INRS :
DANGER! LIQUIDE ET VAPEURS TRES INFLAMMABLES. MORTEL EN CAS D’INGESTION ET PENETRATION PAR VOIE RESPIRATOIRE. IRRITATION CUTANEE. RISQUES AVERES D’EFFETS GRAVES POUR ORGANES. TOXIQUE POUR ORGANISMES AQUATIQUES.
Risques pour la santé et l’environnement liés à l’exposition à l’hexane

Inflammable

Danger grave pour la santé

Risque sanitaire

Dangereux pour l’environnement
L’hexane est classé comme substance dangereuse par l’OSHA (Occupational Safety and Health Administration) selon la norme de communication sur les risques (29 CFR 1910.1200).
En Europe, l’ECHA (Agence européenne des produits chimiques) indique que l’hexane peut endommager les organes en cas d’exposition prolongée ou répétée et qu’il est suspecté d’altérer la fertilité ou de nuire à l’enfant à naître.
In Europe, the ECHA (European Chemical Hazards Agency) highlights that this substance causes damage to organs through prolonged or repeated exposure and is suspected of damaging fertility or the unborn child.
L’hexane est-il produit de manière durable ?
Non — l’hexane n’est pas produit durablement. Il est dérivé du pétrole, comme le charbon et le gaz naturel, des ressources non renouvelables. Le pétrole a mis des millions d’années à se former, et son extraction puis son utilisation rejettent du carbone fossile dans l’atmosphère. Or, nous consommons ces ressources beaucoup plus vite qu’elles ne se renouvellent, ce qui rend leur remplacement impossible à court terme.
C’est pourquoi de nombreux industriels cherchent aujourd’hui à remplacer les produits issus du pétrole et des solvants chimiques par des alternatives végétales et durables. Ils privilégient ainsi des matériaux éco-responsables plutôt que des solutions non renouvelables.
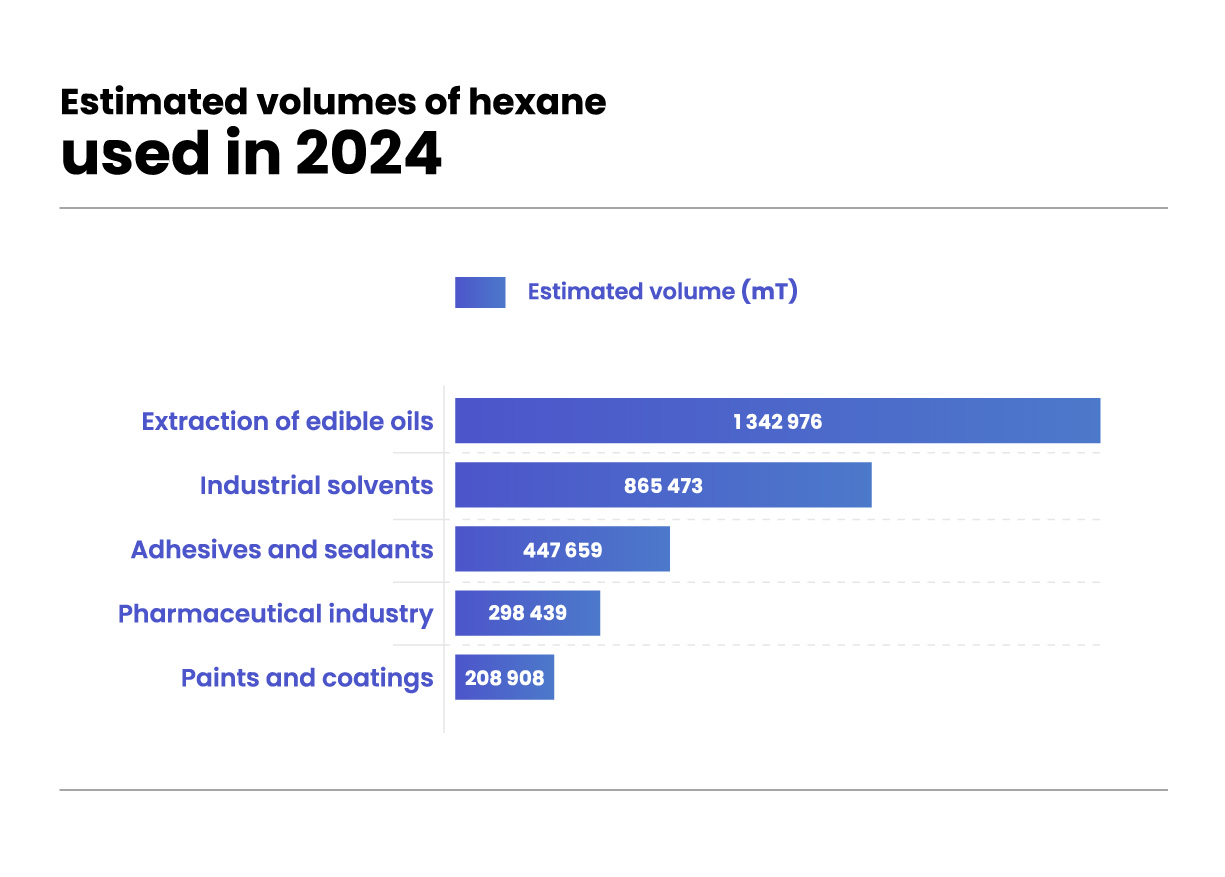
Utilisations de l’hexane
L’usage principal de l’hexane se situe dans l’industrie agroalimentaire, où il sert à extraire les huiles végétales des graines, à dégraisser les protéines destinées à l’alimentation humaine et animale, ou encore comme agent nettoyant dans l’impression, le textile, l’ameublement et la fabrication de colles spéciales (y compris en cordonnerie).
Il est aussi utilisé dans l’extraction d’arômes, de parfums, de compléments nutritionnels et de substances pharmaceutiques à partir de matières biologiques.
En raison de sa facilité d’accès, les solvants et colles contenant du n-hexane sont parfois détournés pour un usage abusif par inhalation.
Dans l’alimentation, le n-hexane est surtout utilisé pour extraire l’huile des graines végétales. Ce procédé fournit deux principaux produits : l’huile, largement employée dans les applications alimentaires, et les protéines déshuilées (dégraissées), utilisées à la fois pour l’alimentation humaine et animale.
Concrètement, les graines sont broyées et mélangées à l’hexane pour en extraire l’huile et obtenir des tourteaux déshuilés (protéines dégraissées), qui constituent une base importante de l’alimentation animale. L’hexane est ensuite retiré des produits finis puis recyclé.
Cependant, une partie de l’hexane n’est pas totalement éliminée lors du recyclage : une fraction s’échappe dans l’atmosphère et une autre reste sous forme de résidus dans les produits. Lorsque les tourteaux contenant ces résidus sont utilisés comme aliments pour le bétail, on peut ensuite en retrouver des traces dans la viande, le lait ou les œufs.
Ainsi, la plupart des aliments transformés de manière conventionnelle — produits de boulangerie, plats préparés, huiles de table, préparations infantiles, produits à base de plantes, et potentiellement lait, œufs et viande — peuvent contenir des résidus d’hexane. L’hexane est également employé dans l’extraction de certains arômes naturels.
If Hexane is so Toxic, why is it still being used in the Food and Feed Processing?
There are three key reasons that neurotoxic hexane is still being used in the extraction of products such as vegetable oils, fats, flavors, fragrances, color additives or other bioactive ingredients.
What Regulations control the use of Hexane?
Hexane, classified as a processing aid, is subject to less strict regulations than food additives and does not require labeling. While international guidelines, such as the Codex Alimentarius, place responsibility on suppliers and users to demonstrate its safety, no regulatory agency has yet set a safe ingestion dose. A 2024 EFSA review has called for a reexamination of its authorization in food processing.
- HOW HEXANE IS PRODUCED
- HEXANE CURRENT AND FUTURE REGULATIONS
- EDIBLE OIL EXTRACTION TIMELINE
- SUSTAINABLE ALTERNATIVES TO HEXANE EXTRACTION
To find out the impact of hexane on human health :