HOW HEXANE IS PRODUCED
From Petroleum to Processing and its Environmental impact
What exactly is hexane, and how is it produced? As this petroleum-based solvent remains widely used for economic reasons, it’s time to clarify its origin, understand its environmental impact, and question whether its continued use is still justified in light of today’s health and sustainability challenges.
Hexane production
What is the primary source?
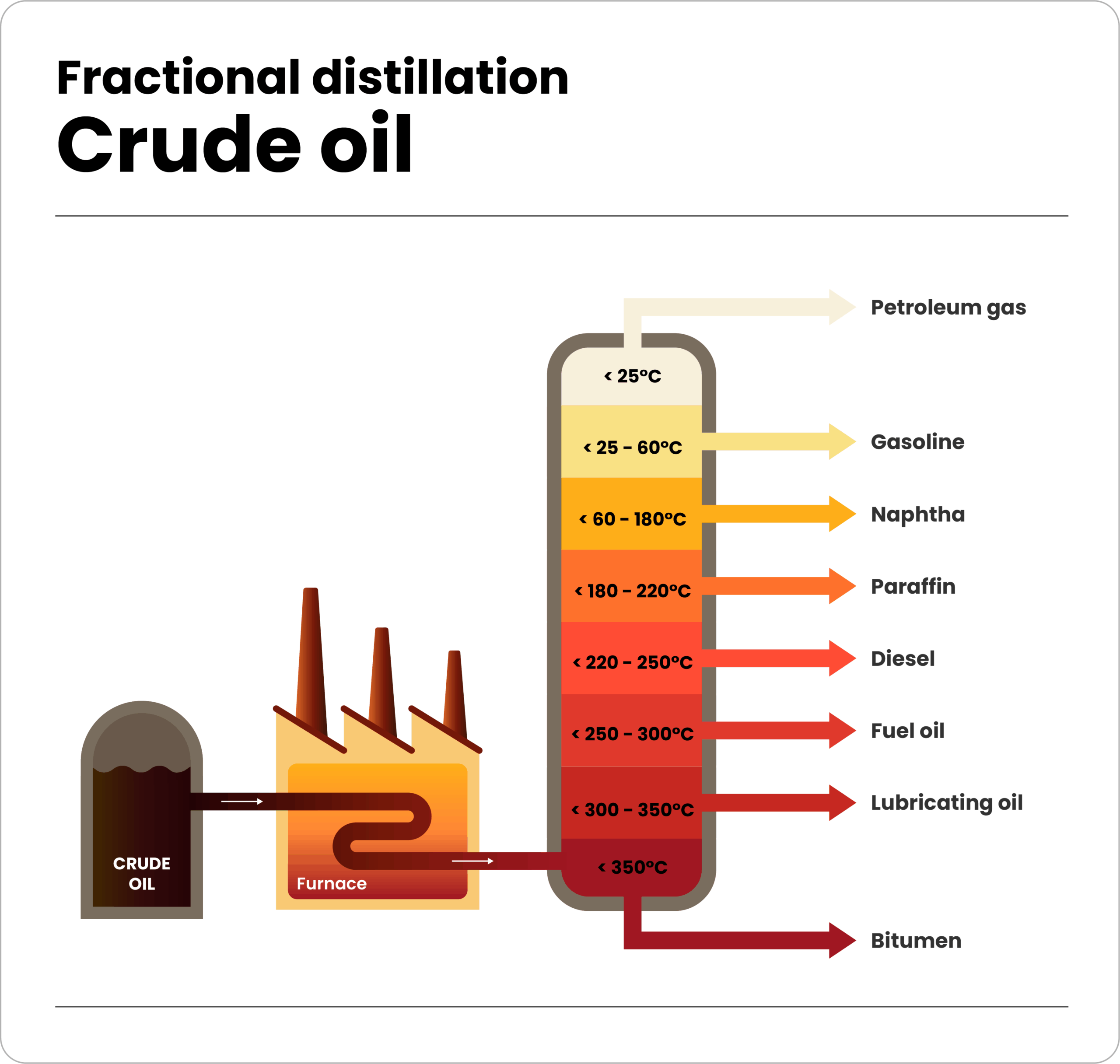
Hexane is primarily produced through the refining of crude oil, specifically by a process called fractional distillation, where the crude oil is heated and separated into different fractions based on their boiling points.
The hexane fraction is being collected at the temperature range where hexane vaporizes; essentially, it is extracted from the naturally occurring hydrocarbon mixture found in petroleum, making it a byproduct of the oil refining process.
Fractional distillation
The key step in hexane production is fractional distillation, where crude oil is heated in a distillation tower, causing different hydrocarbon components to vaporize at different temperatures and separate into distinct fractions.
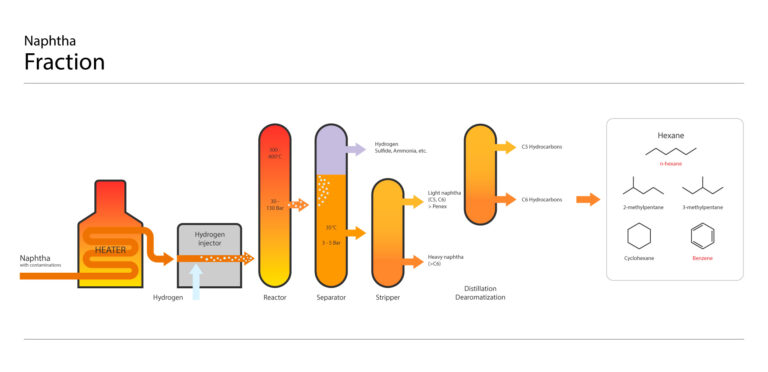
NAPHTA Fraction collection
The fraction “NAPHTA” containing hexane is collected based on its boiling point range, typically around 65-70°C (149-158°F).
NAPHTA purification and distillation to isolate Hexane
Hexane is then isolated from the NAPHTA fraction collection by several successive purification and distillation steps to partially remove impurities like sulfur and aromatic molecules.
Hexane composition
The “hexane” collected is not pure n-hexane; it usually contains a mixture of hexane isomers (different structural arrangements of the same molecule) with n-hexane being the primary component. “Technical hexane” is a mixture of hexane isomers, usually containing more than 60% (n)-hexane.
Environmental damage from hexane production or use in extraction production
The primary environmental damage caused during hexane production or by the use of hexane in extraction is air pollution due to its high volatility.

Because hexane is a highly volatile organic compound (VOC), it can readily evaporate into the atmosphere during production, extraction, refining and purification processes if proper controls are not in place to contain these emissions. While many facilities use systems to capture or reduce volatile organic compounds (VOCs), the regulatory limits currently in force are increasingly being challenged, as there is still a lack of comprehensive toxicological studies to clearly establish safe thresholds that guarantee no harmful effects on health or the environment.
When hexane vapors escape into the air, several things can happen. Because hexane is heavier than air, part of it tends to remain close to the ground, increasing the risk of direct inhalation for people living or working near facilities that produce or use it.
At the same time, hexane present in the atmosphere can react with sunlight and other air pollutants, such as nitrogen oxides, to form photochemical smog. This smog can harm human health, leading to irreversible damage to the lungs and heart, breathing difficulties, and irritation of the respiratory system. Certain crops, like tomatoes and spinach, are also sensitive to smog, which can reduce their growth and cause agricultural losses.
Over time, hexane in the air undergoes photodegradation, gradually breaking down into other compounds, including carbon dioxide (CO₂). This process typically takes about 48 hours but does not fully eliminate the environmental impact, as it still contributes to greenhouse gas emissions.
Understanding these pathways helps explain why emissions from hexane production and use remain an environmental and public health concern.
Hexane’s impact isn’t limited to the air—it can also affect soil and water, with serious consequences for ecosystems and nearby communities.

During production, there’s a risk of spills, leaks, or improper disposal, especially when large volumes of hexane are handled in industrial settings. Hexane can enter soil and groundwater through these incidents or through disposal in landfills.
While hexane is generally not considered persistent in soil or water under normal conditions, significant releases during production can still impact local environments. In certain cases, hexane may persist in these environments, especially when there’s a lack of oxygen or nutrients needed for natural biodegradation.

Hexane is classified as toxic to aquatic life with long-lasting effects. Studies have shown that low to moderate concentrations of n-hexane can be harmful to aquatic life. For example, one study found that n-hexane was borderline highly toxic to the saltwater invertebrate species Chaetogammarus marinus and Mysidopsis bahia.
These contaminations can affect not only local ecosystems but also the health of people living near industrial sites, raising concerns about long-term exposure and environmental justice.